Introduction
This annual report captures the extent and nature of activities undertaken by the National Institutes of Health (NIH) in collaboration with the other agencies and divisions of the Department of Health and Human Services (HHS). Tasked with improving the health of the American public, HHS consists of many agencies and divisions, and collaborations among these different components are vital to the success of the department as a whole. In recognition of the important role of collaborations between HHS agencies, Congress added section 403A(a) of the Public Health Service Act, 42 U.S.C. § 283a(a), Annual Reporting to Increase Interagency Collaboration and Coordination, via Section 104 of the National Institutes of Health Reform Act of 2006. This law mandates that the NIH Director provide to the Secretary of HHS an annual report on NIH’s collaborations with other HHS agencies. This, NIH’s sixteenth report to the Secretary, covers Fiscal Year 2022 (FY22).
Background
The HHS mission is to enhance the health and well-being of all Americans by providing for effective health and human services and by fostering sound, sustained advances in the sciences underlying medicine, public health, and social services. As outlined in the HHS Strategic Plan for FY 2022-2026, the Department sets forth five interrelated, strategic goals to achieve this mission:
- Protect and Strengthen Equitable Access to High Quality and Affordable Healthcare
- Safeguard and Improve National and Global Health Conditions and Outcomes
- Strengthen Social Well-Being, Equity, and Economic Resilience
- Restore Trust and Accelerate Advancements in Science and Research for All
- Advance Strategic Management to Build Trust, Transparency, and Accountability
HHS accomplishes its mission and meets its strategic goals and associated objectives, strategies, and performance goals through the activities of its twelve operating divisions, including nine agencies in the U.S. Public Health Service and three human service agencies, which administer HHS’ multifaceted programs and initiatives. In addition, staff divisions of the Office of the Secretary provide leadership, direction, and policy guidance to the Department. Together, this ‘HHS Family’ covers a vast spectrum of activities that affect health, public health, and human services outcomes. With more than 100 programs across the Department, the ultimate success of all components of the HHS family is enhanced by interagency collaborations that enable agencies to combine their knowledge and diverse expertise to accomplish their collective mission. This cross-agency teamwork is necessary to create a collaborative community within HHS that accelerates progress in medicine, health services, and public health programs.
As the largest research arm of HHS, NIH’s mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability, which it fulfills through its congressionally mandated NIH-Wide Strategic Plan for FY 2021-2025. NIH’s collaborative efforts with other HHS agencies are vital to transforming fundamental scientific and technical information into effective, knowledge-based approaches that advance the health and safety of the public, such as disease treatments, preventive interventions, protective health policies and regulations, and public health campaigns. In turn, the information provided by other HHS agencies on public health needs informs NIH research policies and priorities.
The interagency collaborations included in this report cover joint activities undertaken by NIH with all other components of HHS, including the staff divisions within the Office of the Secretary¹ and the eleven operating divisions of HHS:
- Administration for Children and Families (ACF)
- Administration for Community Living (ACL)
- Agency for Healthcare Research and Quality (AHRQ)
- Administration for Strategic Preparedness and Response (ASPR)
- Agency for Toxic Substances & Disease Registry (ATSDR)
- Centers for Disease Control and Prevention (CDC)
- Centers for Medicare & Medicaid Services (CMS)
- Food and Drug Administration (FDA)
- Health Resources and Services Administration (HRSA)
- Indian Health Service (IHS)
- Substance Abuse and Mental Health Services Administration (SAMHSA)
FY22 Collaborations
NIH and other HHS operating, and staff divisions reported 822 collaborative activities in FY22. These cross-agency collaborations demonstrate the complex array of health efforts that NIH contributes to in partnership with the rest of the Department and can be organized into six overarching themes:
- Assessing the Public’s Health – enabling better tracking of disease and disability
- Improving Diagnostics and Treatment – promoting research, and the translation of NIH’s research results into safe and effective diagnostics and treatments
- Preventing Disease and Disability – providing the evidence base for national disease and disability prevention efforts
- Providing Evidence-Based Health Information – equipping public health efforts and the American public with the latest research findings and best available health information
- Keeping Americans Safe – ensuring effective health policy and regulatory protections
- Broad, Multi-Purpose Coordination – coordinating complex strategic planning efforts that cut across the entire Department
Figure 1: Advancing the public’s health through the integration of the six thematic Areas of NIH’s Collaborations with Other Agencies of the Department of Health and Human Services demonstrate the broad spectrum of health efforts that NIH contributes to in partnership with the rest of the Department.
FY22 Collaborative Activities by the Numbers
In FY22, NIH reported 822 collaborations with other HHS entities as depicted below in Chart 1. Fifty-one new collaborations were reported in FY22, across a range of issues, including activities devoted to protecting the American people from the novel coronavirus, improving healthcare quality and safety, expanding knowledge, and stimulating innovation.
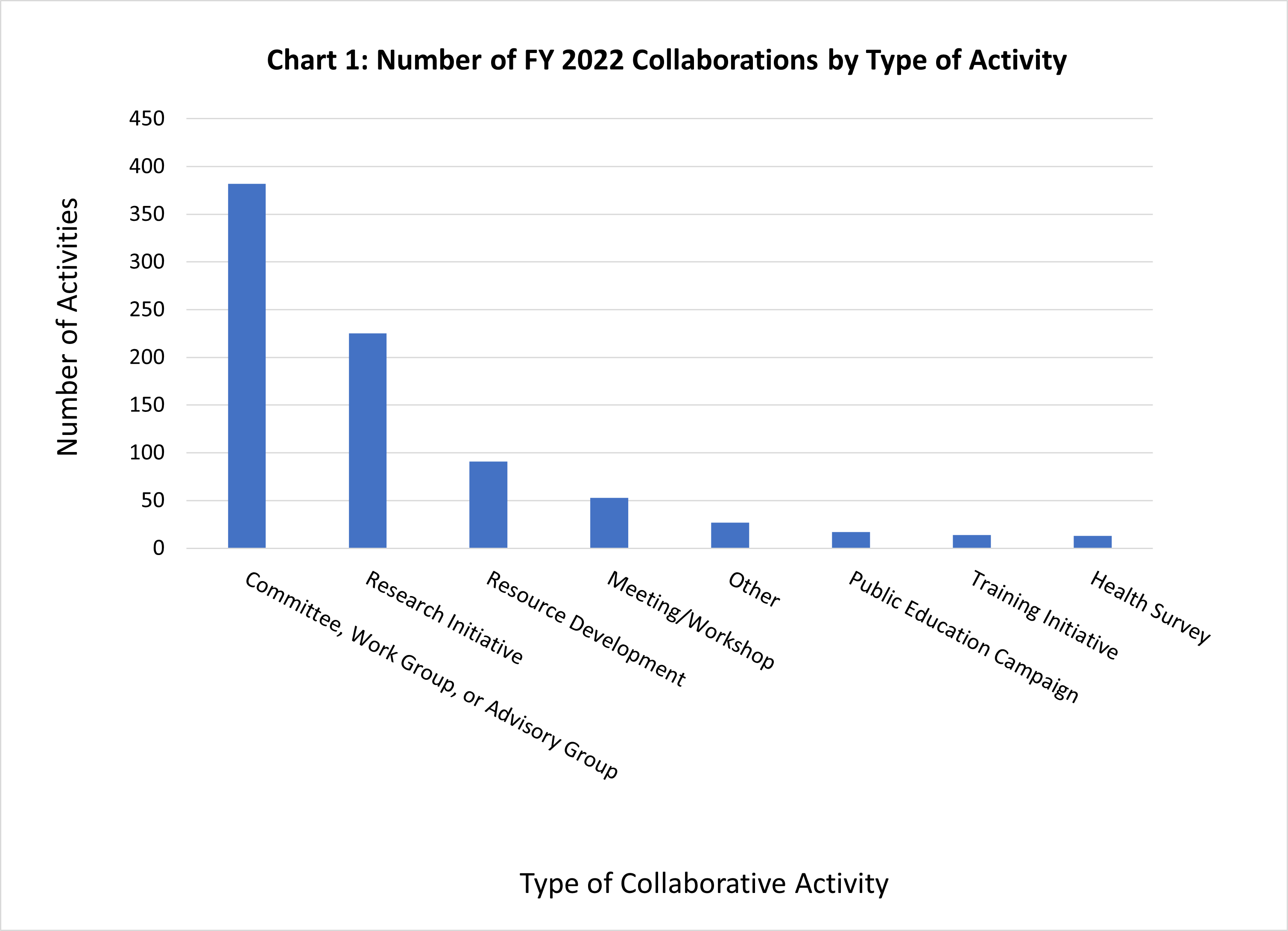
Each collaborative activity has been categorized based on the nature of the activity. Chart 1 illustrates how the activities break down across the following categories: Committee, Advisory Group, or Work Group (382); Research Initiative (225); Resource Development, (e.g., developing databases, disease registries, and information clearinghouses) (91); Meeting or Workshop (53); Other (27); Public Education Campaign (17); Training Initiative (14); or Health Survey (13).
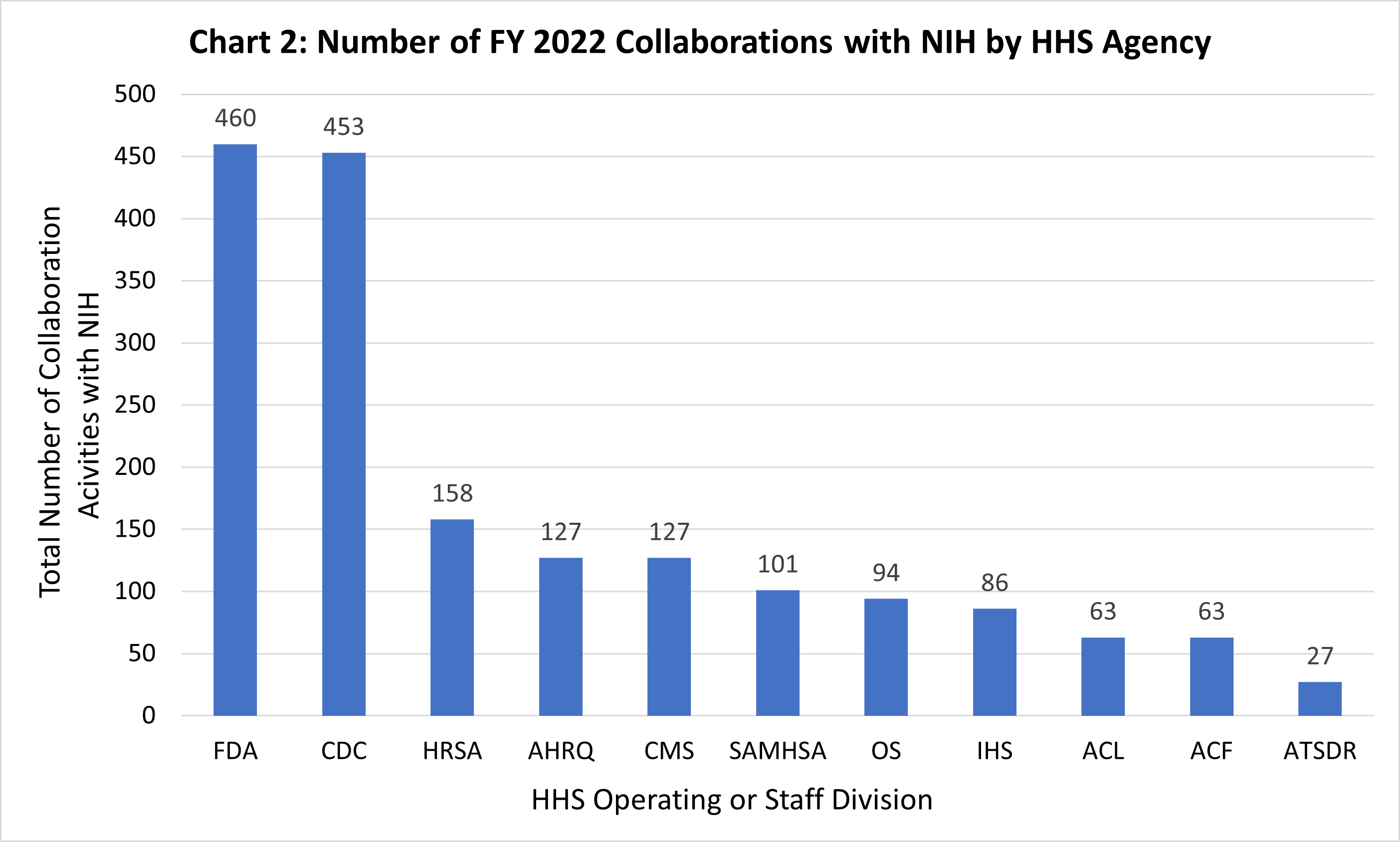
Chart 2 displays the number of reported collaborative activities that NIH engaged in with each HHS operating or staff division in FY22.² As Chart 2 illustrates, the majority of NIH’s collaborations were with FDA (460) and CDC (453). Given the complementary missions of CDC, FDA, and NIH, the three agencies often work closely together to build on each other’s strengths and achieve shared objectives. While NIH conducts and funds basic and applied biomedical and behavioral research, CDC engages in health promotion, prevention of disease, injury and disability, and preparedness for new health threats. The FDA ensures the safety of drugs, vaccines, medical devices, and many other health products that stem from biomedical research. There are also substantial collaborations between NIH and the Office of the Secretary, especially in the coordination of multi-agency initiatives, committees, and working groups.
FY22 Collaboration Highlights
In FY22, HHS published the HHS Strategic Plan for FY 2022-2026. As described above, it outlines five strategic goals for the Department. NIH works closely with its sister agencies to address each of these goals in a myriad of ways. These collaborative activities also contribute toward advancing the priorities set forth in the NIH-Wide Strategic Plan for FY 2021-2025. The following examples of NIH activities describe some of the efforts undertaken in each of the Department’s strategic goal areas.
Protect and Strengthen Equitable Access to High Quality and Affordable Healthcare
NIH supports the Department’s priorities on improving access to healthcare systems and resources through collaborations with HRSA, CDC, SAMHSA, ASPE, and CMS among other agencies. The Federal Cervical Cancer Collaboration (FCCC) is a collaboration between HRSA, CDC, and NIH’s National Cancer Institute and Office for Research on Women's Health. The FCCC aims to accelerate uptake of new recommendations for cervical cancer screening and management. It also focuses on reducing the persistent disparities of this screening through more equitable HPV vaccination among geographically isolated and economically and medically vulnerable populations. In FY22, the FCCC hosted roundtable discussions with several groups including primary care providers, oncologists, patient navigators and advocates, researchers, and members of government, to develop toolkits especially for safety-net settings of care that serve low-income, uninsured and underinsured populations.
NIH also participates in the Office of National Drug Control Policy Harm Reduction Interagency Workgroup (IWG), with other HHS agencies including SAMHSA, ASPE, CMS, HRSA, and CDC. Launched in FY22, this IWG supports the Biden-Harris Administration goal of reducing opioid overdose by increasing access to evidence-based harm reduction services—which include community-based prevention, treatment, and recovery support—through expanded Medicaid reimbursement, comprehensive federal grant funding, research, and workforce training for these services. Specifically, the IWG focuses on integrating harm reduction services with traditional health care infrastructure by developing sustainable funding streams needed to expand the availability of syringe services programs, naloxone, fentanyl test strips, and low-threshold medication treatment for opioid use disorder through harm reduction programs, as outlined in the 2022 National Drug Control Strategy.
Through its participation in the Collaborative on Healthy Parenting in Primary Care, NIH supports additional prevention efforts that contribute to both this strategic goal of providing improved access to healthcare resources, as well as to Secretary Becerra’s 2022 priority of improving child well-being. This collaborative recognizes the effectiveness of family-focused prevention programs that promote the physical and behavioral health and emotional well-being of children from before birth through adolescence. It supports the integration of effective programs that promote healthy parenting into primary care settings to achieve optimal health for children. Members of the Collaborative—including NIH, CDC, HRSA, and SAMHSA—have developed four primary aims to: 1) increase the research evidence supporting the effectiveness of family-focused preventive interventions in primary care settings; 2) increase public awareness and strengthen political will for this initiative; 3) increase the acceptance of these preventive interventions by health care professionals; and 4) identify and develop sustainable funding mechanisms to cover the costs of these services.
Safeguard and Improve National and Global Health Conditions and Outcomes
Continuing efforts to mitigate the risk posed by COVID-19 remains central to safeguard and improve national and global health. By leveraging established—and developing new—partnerships NIH and its sister HHS agencies were able to work with other government and private partners to accelerate the development and implementation of strategies to diagnose, prevent, treat, and prevent this disease. In FY22, one such collaboration, the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership, continued to develop research strategies for prioritizing and speeding development of the most promising treatments and vaccines. Coordinated by the Foundation for the National Institutes of Health (FNIH), ACTIV brought NIH together with its sister agencies in HHS, including the Biomedical Advanced Research and Development Authority within ASPR, CDC, and FDA; other government agencies including the Department of Defense and Department of Veterans Affairs; White House officials; the European Medicines Agency; and representatives from academia, philanthropic organizations, and numerous biopharmaceutical companies. By the end of FY22, this partnership had evaluated hundreds of available therapeutic agents with potential application for COVID-19, prioritized the most promising candidates, designed and harmonized protocols for ACTIV clinical trials, and selected numerous NIH-supported networks to launch these clinical trials to test prioritized therapeutic candidates. Examples of the ACTIV trials included:
- The ACTIV-1 protocol tested three promising immune modulator compounds, a class of drugs that help minimize the deleterious effects of an overactive immune response to SARS-CoV-2 infection. This Phase 3 trial enrolled hospitalized adults with moderate to severe COVID-19 disease. The goal of the trial was to evaluate the safety and efficacy of each immune modulator when given as an add-on therapy to standard of care at local clinics. The different treatments were assessed with respect to illness severity, recovery speed, mortality, and hospital resource utilization. The trial is completed, and results on the two immune modulators that showed efficacy are available to the public.
- ACTIV-6 was designed to test the effectiveness of repurposed drugs (drugs that are FDA-approved for non-COVID-19 indications and have known safety profiles) in reducing the duration and severity of symptoms associated with mild-to-moderate COVID-19. The large, randomized, placebo-controlled Phase 3 trial enrolled outpatient participants who were at least 30 years old, tested positive for SARS-CoV-2 infection, and showed two or more mild-to-moderate symptoms of COVID-19 for no more than seven days. Results from the trials for ivermectin, fluvoxamine and fluticasone are available to the public.
The Tracking Resistance and Coronavirus Evolution (TRACE) Working Group was initiated in FY21 as a part of ACTIV, continuing its work in FY22. The initiative was designed to develop the infrastructure and processes for monitoring and testing emerging SARS-CoV-2 variants and standardizing, gathering, and sharing variant sequencing data. The initiative surveys for new viral variants, assesses vaccine and therapeutic resistance, and evaluates the impact of genetic variation on viral biology and on the clinical approaches for preventing and treating illness. TRACE publishes a weekly report that summarizes shifting trends in emerging SARS-CoV-2 variants. TRACE uses these data to inform which viral variants should be studied to determine the effectiveness of vaccines; coordinate data sharing; and confirm testing and periodic public reporting of results to allow confident decision making by the U.S. Government, health professionals, and pharmaceutical organizations.
In addition to the health risks posed by COVID-19, NIH remains engaged in collaborations to address other ongoing public health crises such as the national opioid crisis. NIH participates in the Pregnant People, Substance Use Disorder and Child Welfare Sub Interagency Policy Committee (IPC) of the Maternal Health IPC with other HHS agencies including ACF, ASPE, CDC, HRSA, IHS, SAMHSA, OS, and IOS. Launched in FY22, this Sub-ICP addresses the Biden-Harris Administration’s First Year Drug Policy Priority to identify barriers and establish policy to help pregnant people with substance use disorder obtain prenatal care and addiction treatment without fear of child removal. This Sub-IPC provides short- and long-term federal government actions to improve equitable outcomes for families affected by substance use disorder involved in, or at risk of being involved in, the child welfare system.
Strengthen Social Well-Being, Equity, and Economic Resilience
To support HHS’ strategic goal to strengthen social well-being, equity, and economic resilience, NIH is collaborating with other agencies within the department to advance several initiatives forward. One example of this is NIH’s participation with ACF, CMS, ACL, AHRQ, FDA, IHS, ASPE, CDC, and OASH in the Behavioral Health Coordinating Council (BHCC) Children and Youth Behavioral Health Subcommittee. Launched in FY21, and continuing its work into FY22, this subcommittee aims to: 1) ensure children, youth, and caregivers have access to appropriate behavioral health prevention resources, screening, referral, and treatments in their communities, schools, and early education settings; 2) address behavioral health needs resulting from the COVID-19 pandemic; 3) advance health equity by addressing the health needs of underserved communities; and 4) support the behavioral health workforce that serves children and youth.
When articulating the goals for the Department for FY22 and the next five years, HHS committed to reduce health disparities among Americans. NIH participates in the Social Determinants of Health Interagency Policy Committee (SDOH-IPC). Convened by the White House Domestic Policy Council and the Office of Science and Technology Policy, the SDOH-IPC is developing a White House Action Plan to address Social Determinants of Health by improving data accessibility and integration. In addition to participating in the SDOH-IPC, NIH also participates with FDA and AHRQ in the Coordinating Committee on Research on Women's Health (CCRWH) Sex, Gender, and Intersectionality (SG&I) Innovations Working Group. This working group was launched in FY22 to stimulate and accelerate innovation in women’s health research and sex-, gender-, and intersectional-specific interventions; thereby creating scientific, societal, economic opportunities (i.e., entrepreneurial opportunities) that promote gender equity.
Restore Trust and Accelerate Advancements in Science and Research for All
As the nation’s biomedical and behavioral research agency, NIH invests in a wide range of basic, translational, clinical, and applied research. To accomplish this efficiently and effectively, NIH collaborates across the Department to restore trust and accelerate advancements in science and research for all. An ongoing example of this is the Accelerating Medicines Partnership®(AMP), which is a public-private partnership between NIH, FDA, and multiple biopharmaceutical companies, managed by FNIH. AMP was developed to transform the model for efficiently developing new diagnostics and treatments across different diseases and health areas. The Accelerating Medicines Partnership - Schizophrenia (AMP® SCZ) is the first neuropsychiatric project within the AMP program, and it is addressing the critical need for safe and more effective treatments for people with schizophrenia. In FY22, the AMP model of partnership was leveraged to address Alzheimer’s disease; autoimmune and immune-mediated diseases; autoimmune disorders of rheumatoid arthritis and systemic lupus erythematosus (lupus); common metabolic diseases; heart failure; and Parkinson’s disease.
Launched in FY21, the Bridge to Artificial Intelligence (Bridge2AI) is a groundbreaking new program that aims to harness the power of artificial intelligence (AI) and machine learning (ML) for human health. The program is designed to set the stage for widespread adoption of AI/ML to propel biomedical research forward and leverage these tools to address biomedical challenges that go beyond the capacity of human intuition. Bridge2AI brings expertise from across the federal government through collaborations with representatives from the Defense Advanced Research Projects Agency, U.S. Department of Energy, National Science Foundation, National Institute of Standards and Technology, and FDA. Bridge2AI is also collaborating with Department of Energy Office of Science program in Advanced Scientific Computing Research to jointly address important issues and challenges in developing AI and ML methods for privacy-sensitive datasets.
Advance Strategic Management to Build Trust, Transparency, and Accountability
In addition to investing in health-focused policies and programs, it is important for NIH and other components of the Department to work on internal operations to further build trust, transparency, and accountability. For over 10 years, NIH, in collaboration with AHRQ, FDA, CMS, ASPE, and CDC, has led the Common Data Elements Task Force. Launched in 2012, it continues to provide a vital forum for communication and transparency across the Department. In FY22 the task force continued to work to improve interoperability of data across the department and across diseases and health areas.
NIH also collaborates with ASPE and FDA on a project to make Medicaid data more accessible through common data models and provide access points for medical records. This project allows systems to efficiently exchange data and to target and retrieve a single data element rather than receive a document containing a patient’s full record. This collaboration is developing and publishing open-source code to format Medicaid information into two common data model formats and assess the data quality that result from this data re-formatting. Through this effort, NIH is contributing to building transparency and accountability across the Department, as data will be used across systems at different agencies.
NIH also contributes to the Department’s efforts to effectively steward public funds by utilizing the Funding Opportunity Announcement Module (FOAM) platform. FOAM allows NIH Institutes and Centers and HHS operating divisions to develop content for—and perform the needed approvals—to publish funding opportunity announcements. The system is flexible and able to meet the needs for HHS operating divisions. In addition, FOAM serves as a federal shared service supporting other agencies, including the Department of the Interior, the Department of Housing and Urban Development, and the Department of Homeland Security. FOAM achieves economies of scale by preventing the need for individual agencies to develop duplicate systems at a cost much greater than that of developing a unified system.
Conclusions
HHS’ mission is to enhance the health and well-being of all Americans, by providing for effective health and human services and by fostering sound, sustained advances in the sciences underlying medicine, public health, and social services. It accomplishes this through many complex programs and initiatives and collaborations. The American public’s investment in NIH provides the nation with a unique resource—a scientific agency devoted to the creation of a knowledge base needed to conquer the most devastating and common human diseases and to improve health for all. For this knowledge base to have impact, the Department, the entire federal government, and the private sector, must continue to work in concert to cultivate ground-breaking biomedical and behavioral research to ensure that scientific knowledge is translated into evidence-based policies, improved health services and medical intervention delivery, and reliable science-based information that all Americans can use to lead healthier lives.
NIH appreciates the opportunity to report on its collaborations within the HHS. The policies, programs, and regulatory and service activities developed and carried out by HHS operating and staff divisions are some of the most effective means the government can use to improve the health and well-being of its citizens. The collaborative activities detailed in this report illustrate how NIH works across the Department to cultivate partnerships, leveraging the respective strengths of all HHS agencies to support the HHS mission, and strengthen the public health ecosystem.
¹ The staff divisions of the Office of the HHS Secretary (OS) are: the Immediate Office of the Secretary (IOS),
Assistant Secretary for Administration (ASA),
Assistant Secretary for Financial Resources (ASFR),
Assistant Secretary for Global Affairs (ASGA),
Assistant Secretary for Health (OASH),
Assistant Secretary for Legislation (ASL),
Assistant Secretary for Planning and Evaluation (ASPE), ,
Assistant Secretary for Public Affairs (ASPA), Office for Civil Rights (OCR),
Departmental Appeals Board (DAB), , Office of Global Affairs (OGA),
Office of the General Counsel (OGC),
Office of Inspector General (OIG), ,
Office of Medicare Hearings and Appeals (OMHA), the HHS Chief Information Officer, and the
Office of the National Coordinator for Health Information Technology (ONC).
² Individual collaborative activities can involve multiple HHS agencies. Therefore, the values displayed in Chart 2 reflect duplicate counts and add up to more than the total reported sums.