Introduction
This annual report captures the extent and nature of activities undertaken by the National Institutes of Health (NIH) in collaboration with the other agencies and divisions of the Department of Health and Human Services (HHS). Tasked with improving the health of the American public, HHS consists of many agencies and divisions, and synergy between these different components is vital to the success of the whole. In recognition of the important role of collaboration between HHS agencies, Congress added section 403A(a) of the Public Health Service Act, 42 U.S.C. § 283a(a), Annual Reporting to Increase Interagency Collaboration and Coordination, via Section 104 of the National Institutes of Health Reform Act of 2006. This law mandates that the NIH Director provide to the Secretary of HHS an annual report on NIH’s collaborations with other HHS agencies. This, our thirteenth report to the Secretary, covers fiscal year (FY) 2019.
Background
The HHS mission is to enhance the health and well-being of all Americans by providing for effective health and human services and by fostering sound, sustained advances in the sciences underlying medicine, public health, and social services. As outlined in the HHS Strategic Plan FY 2018-2022, the Department sets forth five interrelated, strategic goals to achieve this mission:
- Reform, Strengthen, and Modernize the Nation’s Healthcare System
- Protect the Health of Americans Where They Live, Learn, Work, and Play
- Strengthen the Economic and Social Well-Being of Americans Across the Lifespan
- Foster Sound, Sustained Advances in the Sciences
- Promote Effective and Efficient Management and Stewardship
HHS accomplishes its mission and meets its strategic goals and associated objectives, strategies, and performance goals through the work of its eleven operating divisions, including eight agencies in the U.S. Public Health Service and three human service agencies, which administer HHS’s multifaceted programs and initiatives. In addition, staff divisions of the Office of the Secretary provide leadership, direction, and policy guidance to the Department. Together, this ‘HHS Family’ covers a vast spectrum of activities that affect health, public health, and human services outcomes. With more than 115 programs across the Department, the ultimate success of all components of the HHS family is enhanced by interagency collaborations that enable agencies to combine their knowledge and diverse expertise to accomplish their collective mission. Such cross-agency teamwork is necessary to create a collaborative community within HHS that accelerates progress in medicine, health services, and public health programs.
As the largest research arm of HHS, NIH’s mission is “to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability,” which it fulfills through its congressionally mandated NIH-Wide Strategic Plan, Fiscal Years 2016–2020: Turning Discovery Into Health. NIH’s collaborative efforts with other HHS agencies are vital to transforming fundamental scientific and technical information into effective, knowledge-based approaches that advance the health and safety of the public, such as disease treatments, preventive interventions, protective health policies and regulations, and public health campaigns. In turn, the information provided by other HHS agencies on public health needs informs the policies and priorities of NIH-funded research.
The interagency collaborations included in this report cover joint activities undertaken by NIH with all other components of HHS, including the staff divisions within the Office of the Secretary (OS)1 and the ten other operating divisions of HHS:
- Administration for Children and Families (ACF)
- Administration for Community Living (ACL)
- Agency for Healthcare Research and Quality (AHRQ)
- Agency for Toxic Substances & Disease Registry (ATSDR)
- Centers for Disease Control and Prevention (CDC)
- Centers for Medicare & Medicaid Services (CMS)
- Food and Drug Administration (FDA)
- Health Resources and Services Administration (HRSA)
- Indian Health Service (IHS)
- Substance Abuse and Mental Health Services Administration (SAMHSA)
FY 2019 Collaboration Highlights
NIH and other HHS operating, and staff divisions reported 563 collaborative activities in FY 2019. These cross-agency collaborations demonstrate the complex array of health efforts that NIH contributes to in partnership with the rest of the Department and can be organized into six overarching themes:
- Assessing the Public’s Health – enabling better tracking of disease and disability
- Improving Diagnostics and Treatment – promoting research, and the translation of NIH’s research results into safe and effective diagnostics and treatments
- Preventing Disease and Disability – providing the evidence base for national disease and disability prevention efforts
- Providing Evidence-Based Health Information – equipping public health efforts and the American public with the latest research findings and best available health information
- Keeping Americans Safe – ensuring effective health policy and regulatory protections
- Broad, Multi-Purpose Coordination – coordinating complex strategic planning efforts that cut across the entire Department
Examples of collaboration activities from each thematic area are highlighted in Figure 1 and examples of specific collaborations are described in the “FY 2019 Collaboration Highlights” section to follow.
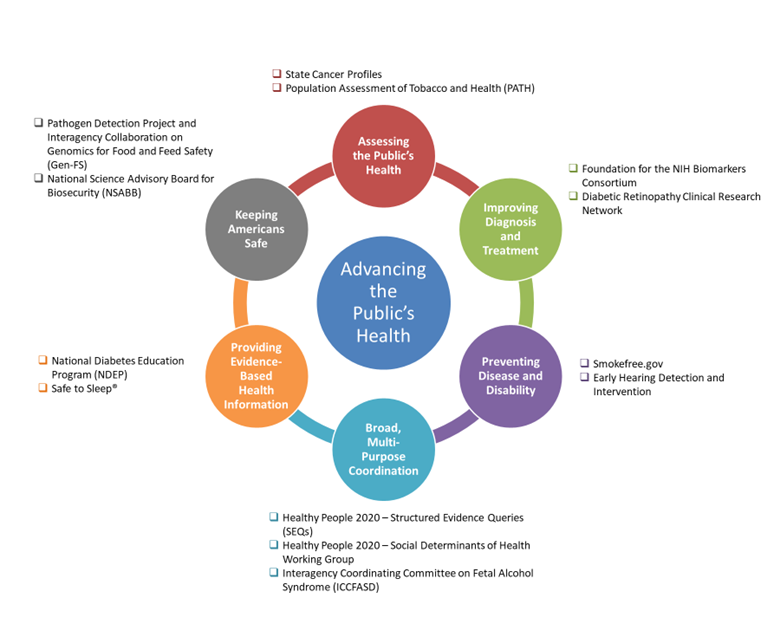
Figure 1: Advancing the Public’s Health: Thematic Areas of NIH’s Collaborations with Other Agencies of the Department of Health and Human Services. Two examples of collaborations within these domains are named here and described in detail below. These activities of cross-agency collaboration demonstrate the broad spectrum of health efforts that NIH contributes to in partnership with the rest of the Department.
FY 2019 Collaborative Activities by the Numbers
In FY 2019, NIH reported 563 collaborations with other HHS entities as depicted below in The Complete List of Activities. Forty-six new collaborations were reported in FY 2019, across a range of issues, including activities devoted to improving healthcare quality and safety, knowledge, and innovation.
Each collaborative activity has been categorized based on the nature of the activity. Chart 1 illustrates how the activities break down across the following categories: Committee, Advisory Group, or Work Group (280 activities); Research Initiative (107); Resource Development, (e.g., developing databases, disease registries, and information clearinghouses) (68); Meeting or Workshop (46); Public Education Campaign (15); Health Survey (10); Training Initiative (10); or Other (27).
Chart 2 displays the number of reported collaborative activities that NIH engaged in with each HHS operating or staff division.2 As Chart 2 illustrates, the majority of NIH’s collaborations were with CDC (335) and FDA (287). Given the complementary missions of CDC, FDA, and NIH, the three agencies often work together closely to build on each other’s strengths and achieve shared objectives. While NIH conducts and funds basic and applied biomedical and behavioral research, CDC engages in health promotion, prevention of disease, injury and disability, and preparedness for new health threats, and FDA ensures the safety of drugs, medical devices, and many other products that stem from biomedical research. There are also substantial collaborations between NIH and the OS, especially in the coordination of multi-agency initiatives, committees, and working groups.
FY 2019 Collaboration Highlights
The following summaries illustrate how NIH collaborates with the other HHS agencies to help improve the health and well-being of the American public. Introduced in Figure 1 (above), examples of specific collaborations in each of the six highlighted themes are described in greater detail in this section. Ultimately, these diverse collaborative efforts strengthen the HHS ecosystem, helping to foster a healthier country and a healthier world.
The Population Assessment of Tobacco and Health (PATH) Study is the first large-scale NIH and FDA collaboration since Congress granted FDA the authority to regulate tobacco products in the Family Smoking Prevention and Tobacco Control Act (FSPTCA) in 2009. The PATH Study is a national longitudinal cohort study that will follow an estimated 59,000 U.S. household residents ages 12 years and older for at least three years. Objectives are to assess initiation and use patterns; to study trends in tobacco-product use cessation and relapse; to monitor behavioral and health impacts, including in risk perceptions and other tobacco-related attitudes; and to assess differences in tobacco-related attitudes, behaviors, and health outcomes among racial/ethnic, gender, and age subgroups. The PATH Study will also collect biospecimens from adults to analyze biomarkers of tobacco use and related health outcomes. By measuring and accurately reporting on the social, behavioral, and health effects associated with tobacco-product use in the U.S., the PATH Study will provide an empirical evidence base to help inform FDA’s decisions about changes in tobacco products in meeting the objectives of the 2009 FSPTCA.
State Cancer Profiles website is a publicly accessible resource that characterizes the burden of different types of cancer in each state in a dynamic and standardized manner in order to motivate action, integrate surveillance into cancer control planning, characterize areas and demographic groups, and expose health disparities. The focus is on cancer sites with evidence-based control interventions. Interactive graphics and maps provide visual support for deciding where to focus cancer control efforts.
The Foundation for the NIH Biomarkers Consortium is a public-private biomedical research partnership managed by the Foundation for the National Institutes of Health (FNIH) that endeavors to discover, develop, and qualify biological markers (biomarkers) to support new drug development, preventive medicine, and medical diagnostics. Biomarkers are objectively measurable indicators of normal and abnormal biological states, such as, for example, hemoglobin A1C as a biomarker of blood sugar control in diabetics. The Biomarkers Consortium, with NIH, FDA, CMS, and private sector members, is looking to rapidly identify, develop, and qualify potential high-impact biomarkers to enable improvements in drug development, clinical care, and regulatory decision-making in areas as diverse as Alzheimer’s disease, cardiovascular disease, diabetes, and breast cancer. For example, in 2018 the Consortium announced that FDA had qualified a new biomarker for use in Phase 1 clinical trials to detect acute kidney injury by investigational drugs, helping to improve the development of safe and effective drugs.
Diabetic Retinopathy Clinical Research Network (DRCR.net), a collaborative network dedicated to facilitating multicenter clinical research on diabetic retinopathy, diabetic macular edema, and associated conditions. DRCR.net’s mission is to improve the lives of individuals with retinal pathology by supporting research that leads to a better understanding of retinal diseases and advances their treatment. Principal emphasis is placed on clinical trials, but epidemiologic outcomes and other research may be supported as well. The network is funded by NIH, and OS, with the Special Funding Program for Type 1 Diabetes Research.
Smokefree.gov is a website that has been designed through the collaborative efforts of NIH, CDC, and OS to help individuals quit smoking, with the understanding that different people need different resources as they try to quit. The information and professional assistance available on this website can help to support both immediate and long-term needs as individuals become, and remain, a nonsmoker. Smokefree.gov allows individuals to choose the help that best fits their needs. This collaboration also includes a number of smoking cessation text message programs throughout the world from a myriad of different regions including, China, Caribbean, Guam, American Samoa, and Samoa.
NIH has partnered with CDC and HRSA on the Early Hearing Detection and Intervention, an effort targeted towards early identification and diagnosis of hearing loss and intervention services for newborns and infants. Hearing loss can affect a child’s ability to develop communication, language, and social skills. The earlier children with hearing loss start getting services, the more likely they are to reach their full potential. HRSA funds awards to states and healthcare providers to screen newborns and young children for hearing loss. CDC funds states to develop data systems to track hearing loss, while NIH funds research to study early hearing detection and intervention services.
Over 30 million adults in the U.S. have diabetes, a chronic (long-lasting) health condition, which is the seventh leading cause of death in the U.S. Established in 1997, the National Diabetes Education Program (NDEP), is a federally-funded educational program sponsored by NIH and CDC and includes partners at the federal, state, and local levels that work together to improve the treatment and outcomes for people with diabetes, promote early diagnosis, and prevent or delay the onset of type 2 diabetes. NDEP also provides educational resources that are culturally and linguistically appropriate for individuals and groups to better understand the impact of diabetes, influence health outcomes and improve access to quality health care.
The Safe to Sleep® campaign seeks to educate parents and caregivers about ways to reduce the risk of Sudden Infant Death Syndrome (SIDS) and other sleep-related causes of infant death, such as accidental suffocation. Established in 1994, this campaign is sponsored by NIH, CDC, and HRSA and offers print and electronic materials, media resources, toolkits, and a continuing education program for nurses to help spread the word about safe infant sleep. The Safe to Sleep® campaign also partners with organizations to help reach various audiences, including African American, and American Indian/Alaska Native communities, which have the highest SIDS rates of any racial/ethnic group in the United States.
To improve surveillance of disease-causing bacteria and foodborne disease, NIH in collaboration with CDC and FDA, established the Pathogen Detection Project and Interagency Collaboration on Genomics for Food and Feed Safety (Gen-FS). This project is a multi-agency collaboration that is combining data from pathogen outbreaks with other information to determine the major source of contamination. The project is conducted via a centralized system that integrates genetic sequence data for bacterial pathogens obtained from food, the environment, and human patients. A number of public health agencies in the U.S. and internationally are collecting samples from these sources to facilitate active, real-time surveillance of pathogens and foodborne disease. The agencies sequence the samples and submit the data to NIH which analyzes the sequences against others in its database to identify closely related sequences. The aim is to uncover potential sources of contamination by linking isolates from food or the environment to human illness and to quickly report the sequence relationships to public health scientists in order to aid traceback investigations and outbreak response. Collaborating agencies include FDA, CDC, USDA-Food Safety and Inspection Service, and Public Health England.
Enhancing biosafety and biosecurity in health science research is a high priority and requires close collaboration between federal departments and agencies. The National Science Advisory Board for Biosecurity (NSABB), managed by NIH, is a Federal advisory committee chartered to provide advice, guidance, and leadership regarding biosecurity oversight of research yielding new technologies or information with the potential for both benevolent and malevolent applications (dual use research) to all federal departments and agencies with an interest in life sciences research. NSABB also undertakes domestic and international outreach and education efforts to raise awareness of issues related to biosecurity and dual use research of concern, the small subset of life sciences research with the highest potential for yielding knowledge, products, or technology that could be misapplied to threaten public health or national security.
The overarching goals for Healthy People 2020 incorporate a focus on social determinants of health as well as on health outcomes and risk factors. The Healthy People 2020 - Social Determinants of Health Working Group develops broad objectives to identify and mitigate social determinants and ensure their integration across all Healthy People 2020 objectives. NIH is also collaborating with the Office of Disease Prevention and Health Promotion within the Office of the Assistant Secretary for Health to develop a web-based resource for public health information through the creation of structured PubMed searches by means of the Healthy People 2020 - Structured Evidence Queries (SEQs). Each PubMed search is a structured evidence query (SEQ) or "seek" developed by the medical librarians and reviewed by members of the Healthy People 2020 working groups. These defined PubMed searches enable the public health workforce to find evidence-based information related to a specific Healthy People 2020 objective. The SEQs are accessible from the Healthy People 2020 website as well as the PHPartners.org website.
The Interagency Coordinating Committee on Fetal Alcohol Syndrome (ICCFASD) was created in October 1996. Chaired by the NIH, the Committee coordinates federal activities on Fetal Alcohol Syndrome (FAS), and other disorders associated with prenatal alcohol exposure. ICCFASD works to improve communication, cooperation, and collaboration among disciplines and federal agencies that address issues of health, education, developmental disability, research, justice, and social services relevant to FAS and related disorders caused by prenatal alcohol exposure.
Conclusion
HHS accomplishes its mission to enhance and protect the health and well-being of all Americans through the numerous programs and initiatives that cover a vast spectrum of activities that affect health, public health, and human services outcome. America’s investment in NIH provides the nation with a unique resource—a scientific agency devoted to the creation of a knowledge base needed to conquer the most devastating human diseases and disabilities. For this rich knowledge base to improve health, the Department, as well as the entire federal government and the private sector, must work in concert to cultivate ground-breaking research and ensure that scientific knowledge is translated into sound regulations and policies, health services and medical interventions, and information that all Americans can use to lead healthier lives.
NIH appreciates the opportunity to report on its multifaceted collaborations within the HHS. The policies, programs, and regulatory and service activities developed and carried out by HHS operating and staff divisions are some of the most effective means that the government can use to improve the health and well-being of its citizens. The collaborative activities detailed in this report illustrate how NIH works across the Department to cultivate partnerships, leveraging the respective strengths of all HHS agencies to support the HHS mission and strengthen the public health ecosystem.
1 The staff divisions of the Office of the HHS Secretary (OS) are: the Immediate Office of the Secretary (IOS), Assistant Secretary for Administration (ASA), Assistant Secretary for Financial Resources (ASFR), Assistant Secretary for Global Affairs (ASGA), Assistant Secretary for Health (ASH), Assistant Secretary for Legislation (ASL), Assistant Secretary for Planning and Evaluation (ASPE), Assistant Secretary for Preparedness and Response (ASPR), Assistant Secretary for Public Affairs (ASPA), Center for Faith-Based and Neighborhood Partnerships (CFBNP), Departmental Appeals Board (DAB), Office for Civil Rights (OCR), Office of the General Counsel (OGC), Office of Inspector General (OIG), Office of Intergovernmental and External Affairs (IEA), Office of Medicare Hearings and Appeals (OMHA), and the Office of the National Coordinator for Health Information Technology (ONC).
2 Individual collaborative activities can involve multiple HHS agencies. Therefore, the values displayed in Chart 2 reflect duplicate counts and add up to more than the total reported sums.